
◈ Clinical Trial Sample Analysis Institution
An
institution that conducts sample analysis on blood, urine, and other specimens
collected from clinical trial subjects is
designated by the Commissioner of the Ministry of Food and Drug Safety in accordance
with the Pharmaceutical Affairs Act,
the Clinical Trial Sample Analysis Management Standards, and regulations related to
the safety of pharmaceuticals.
◈ Prime Healthcare has been designated as a Clinical Trial Sample Analysis Institution (Immunoassay) as of January 24, 2024, in accordance with relevant laws and regulations.

◈ Corporate Research Institute
Corporate Research Institute refers to a system established to promote and support research and development activities within a company by creating an independent research organization or department. Companies meeting specific criteria can declare and gain recognition from the Korea Industrial Technology Association for their Corporate Research Institute or dedicated research departments. This system is designed to facilitate and enhance a company's research and development efforts by providing various benefits and support for their activities.
◈ Prime Healthcare was recognized as a Corporate Research Institute by the Korea Industrial Technology Association (KOITA) on August 24, 2023.
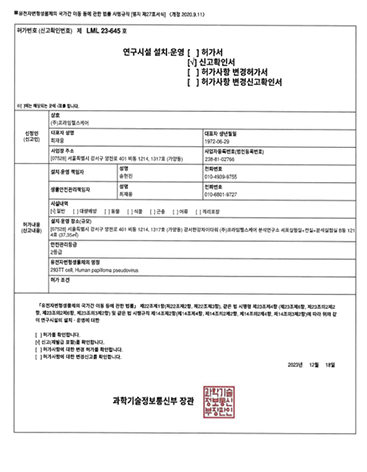
◈ Biological Safety Research Facility
A Biological Safety Research Facility refers to a research facility equipped with a biological safety management system designed to protect human health and the environment, considering the biological hazards posed by organisms, including genetically modified organisms and pathogenic microorganisms.
◈ Prime Healthcare was officially permitted as an LMO (Living Modified Organisms) Research Facility (Grade 2) on December 18, 2023, in accordance with relevant regulations.

◈ ISO 9001
It refers to a quality assurance system spanning the entire production process from products to services. It evaluates and certifies the quality management system that produces and supplies products, allowing suppliers to provide trust to buyers and organizations through objective and independent evaluation by a third-party certification body.
◈ Prime Healthcare Co., Ltd. received ISO 9001:2015 Quality Management System certification from the International Organization for Standardization (ISO) on February 26, 2024.
